Research in the Greb Group
The Greb Research Group explores the influence of strong ligand effects on the reactivity and physicochemical properties of p-block elements to expand the usual reactivity and property profiles of the Earth's most common elements. By dramatically altering the electronic structure through structural constraint or enabling element-ligand cooperativity, new reaction pathways for activating small molecules are discovered. Subtle changes in the oxidation states of p-block elements are achieved by interplay with redox-active ligands (Electromerism). Further, the group pursues a systematic study of the concept of Lewis acidity using data-driven methods. State-of-the-art computational approaches parallel all synthetic projects conducted in our group.
Lewis Acidity
The concept of Lewis acidity was proposed more than 100 years ago. However, based on empiricism, providing a clear-cut parametrization of this concept is problematic. Our group aims to enrich the field of very strong Lewis superacids. More generally, we are working on a reformulation of the theory of Lewis acidity through data science.
For a recent review article, see Chem. Eur. J. 2018, 24, 17881-17896. and Synlett 2024, 35(12), 1382-1398.
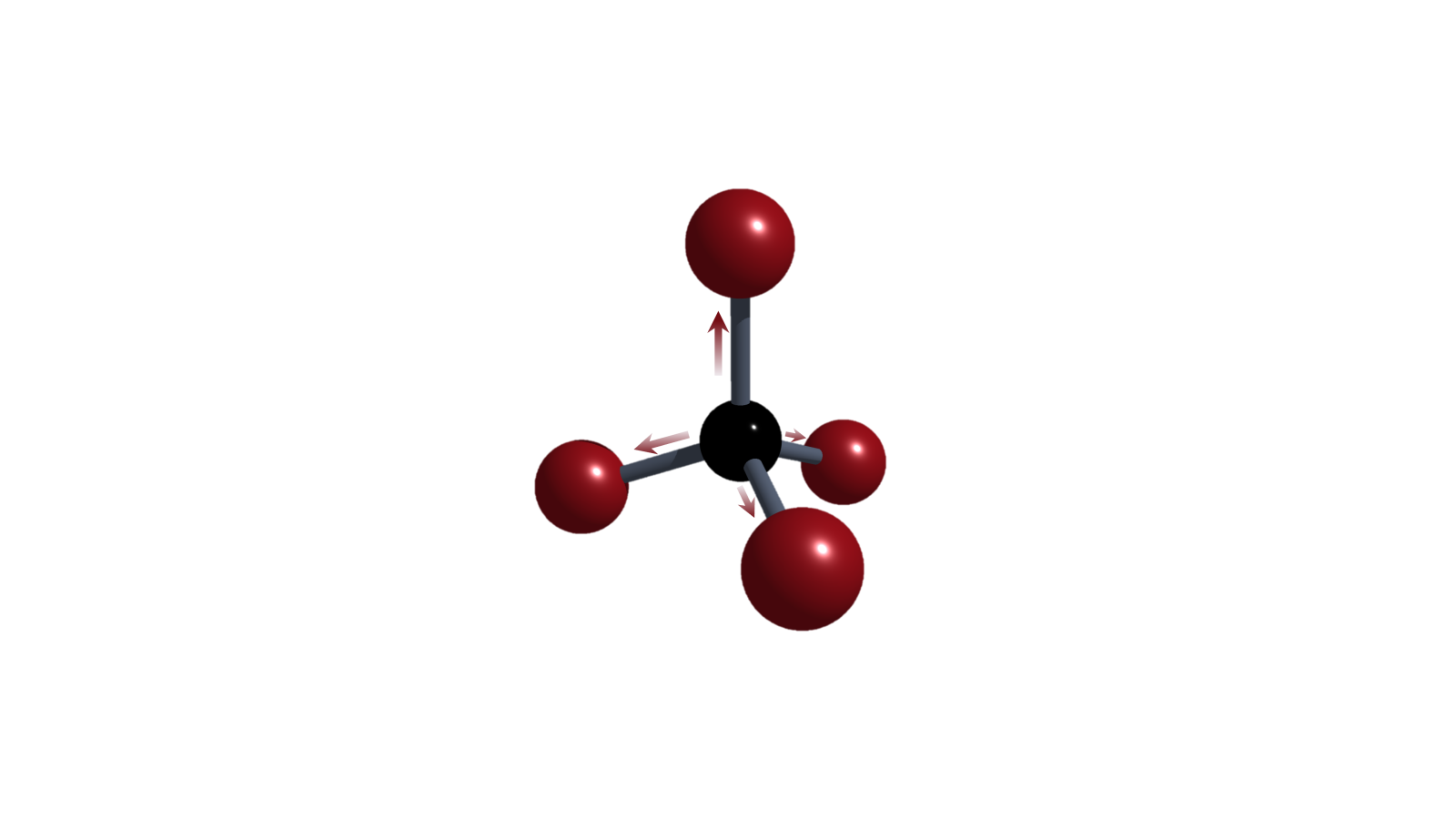
Structural Constraint
Structurally deforming p-block elements into non-VSEPR-compliant geometries is causing substantial changes in the frontier molecular orbital energies and shapes. Consequently, altered reactivities such as increased Lewis acidity or ambiphilicity are unlocked, enabling new transformations. The research is rooted in a fundamental understanding of the theory of molecular deformation and aims to unleash novel concepts for main-group catalysis.
For a recent review article, see Adv. Inorg. Chem. 2023, 82, 261-299.
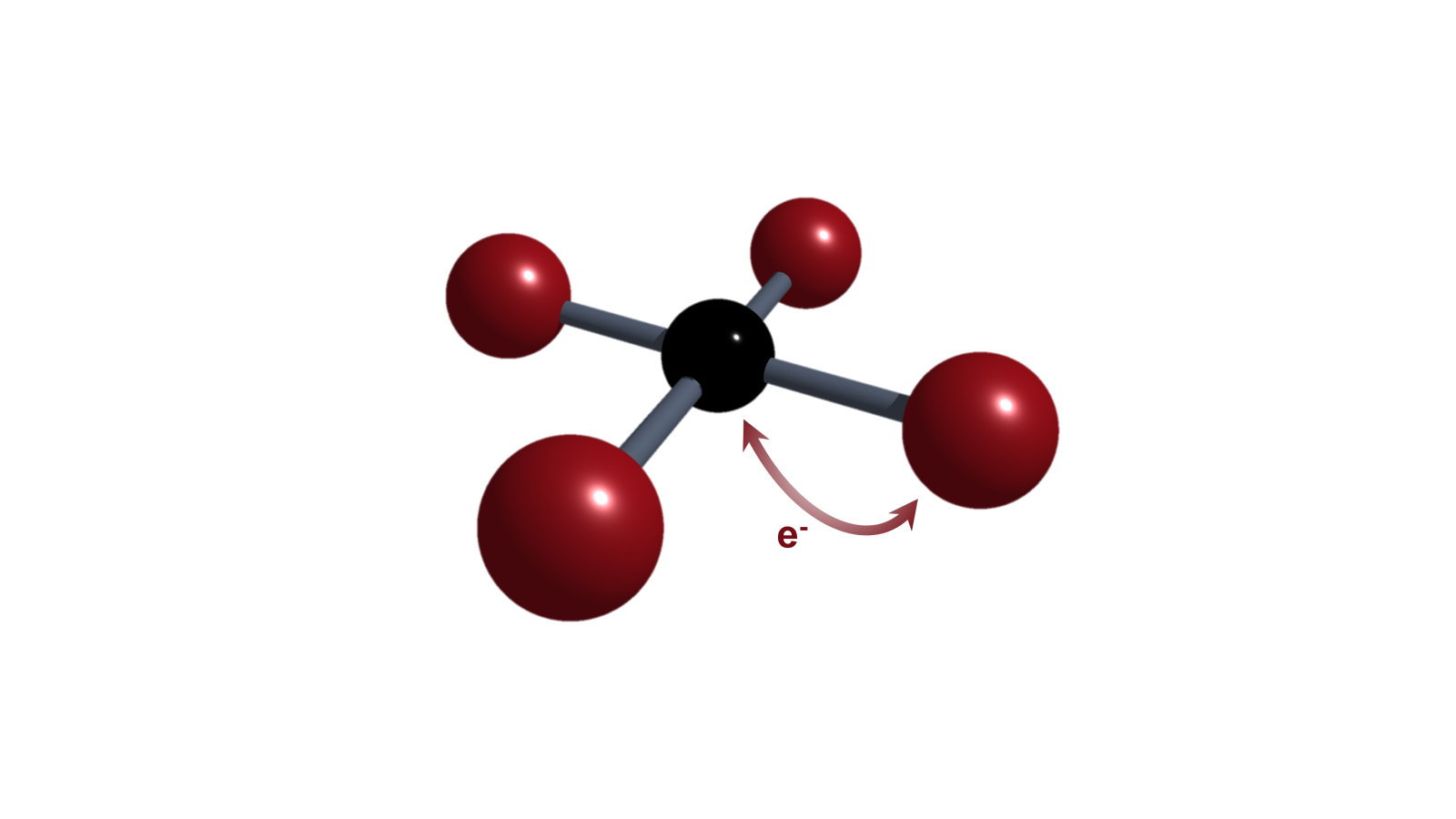
Element-Ligand Cooperativity (ELC)
Ambiphilicity in molecules can be achieved by small HOMO-LUMO gaps. Single-centered ambiphiles (e.g., tetrylenes) or Frustrated Lewis Pairs are prominent candidates for such electronic structures and enhanced reactivity. Our approaches are focused on unusual involvements of the ligand in conjunction with a central p-block element, so-called element-ligand cooperativity (ELC).
For a recent review article, see Eur. J. Inorg. Chem. 2020, 3030-3047.
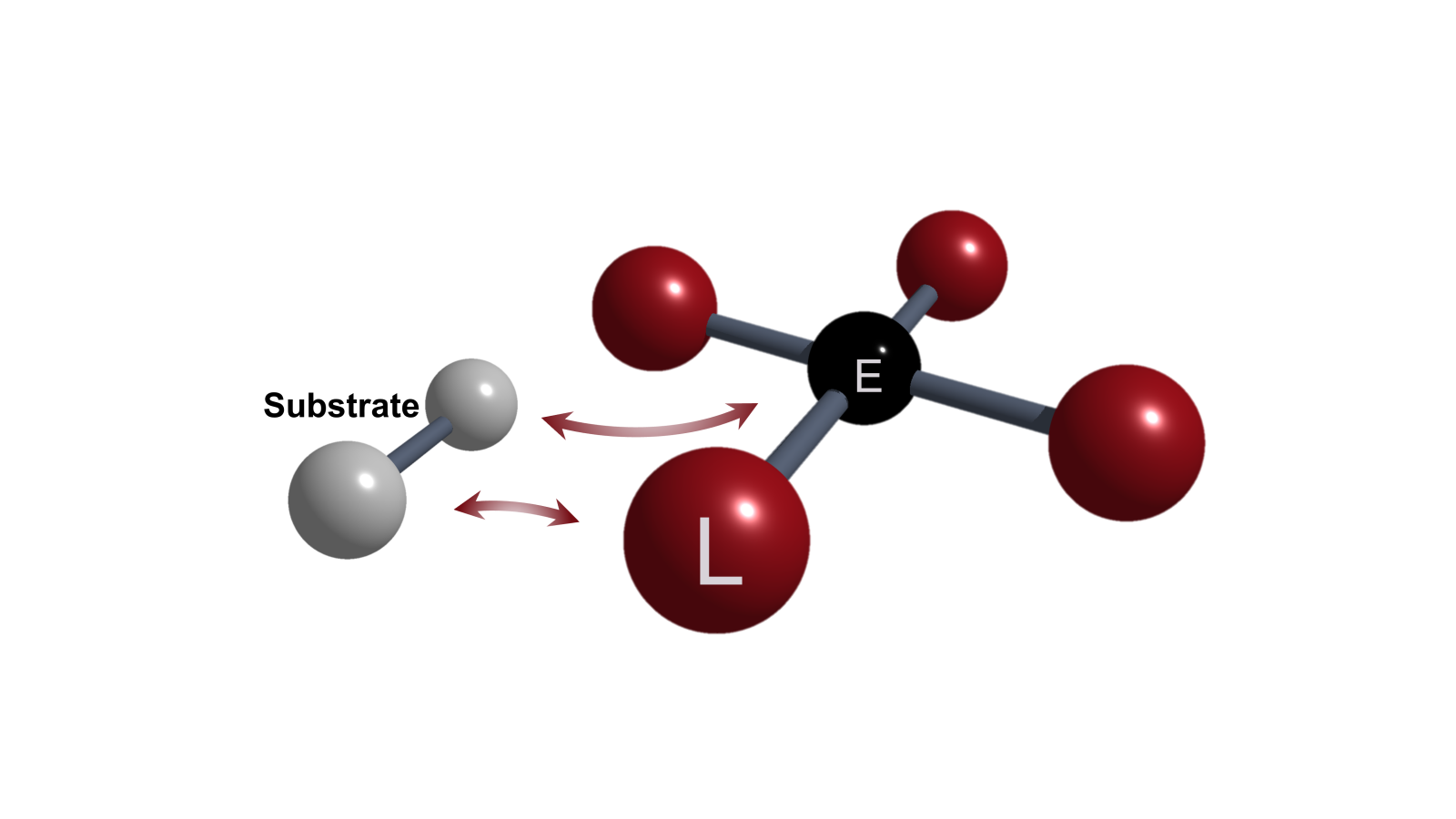
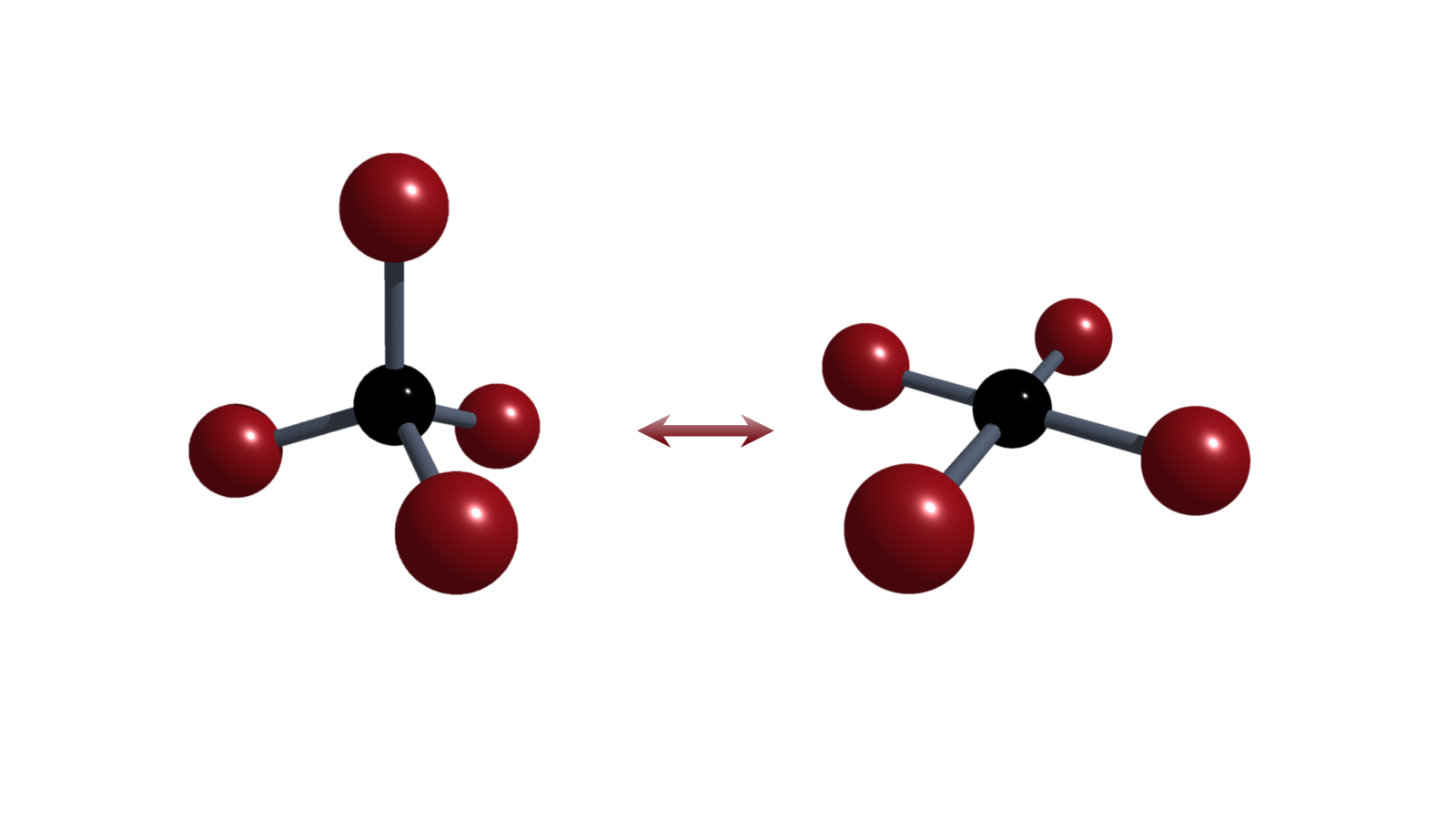
Electromerism
Coupling redox-chemistry of a transition metal with that of a ligand is well established. Our group is interested in expanding this feature, termed electromerism (aka. valence tautomerism), onto p-block elements.
For a recent review article, see Eur. J. Inorg. Chem. 2022, e202100871.